I wanted to do this post for probably around a year now, but just was never quite able to sit down and do it. Surprisingly, now that I am at the busiest I’ve been at the lab, the inspiration finally struck me and I was up until past 2 am squinting into the microscope after hours of microscopy in the lab. Note: don’t do 8 hours of microscopy in one day or your eyes will hate you. By that time I was so tired that quality of the micrographs wasn’t even close to a top priority, so I’ll have to ask your forgiveness if some of the images are even worse than usual.
Anyway, what was it that was on my mind for so long? Vital staining.
There has been a resurgence of interest on the topic among the scientifically slanted homebrewing blogs and, as far as I can tell, everyone out there is using methylene blue to check their yeast viability. It’s not really surprising since MB has long been used as the standard vital stain in the brewing industry and thus adopted by many homebrewers. Another stain often utilized by a wider audience of fermentation and ethanol production industries is methylene violet, which seems to be pretty much the same as methylene blue except for the color. In the science world there are a number of ways to determine viability of the cells ranging from fluorescent dyes to apoptotic/necrotic marker antibodies etc., but such methods require expenses and preparations way out of homebrewing range. The gold standard for non-fluorescent methods, however, is trypan blue. First let’s take a look at some of them.
Methylene Blue – a blue dye that will stain the dead cells blue. Living cells also take in the dye, but the active enzymes within them will process (reduce) the dye and make it colorless. Problems with this stain include that some dead cells still have enough active enzymes left in them to reduce the dye, the dye may block some intracellular processes and result in failure to reduce it by living cells, below pH 4.6 the uptake of the dye increases so that many living cells also become blue, also it may stain stressed live cells with fractured membranes. In my search for more info I found some articles about using various microscopic techniques to tell between the false positive and false negative cells, all of which are beyond the homebrewing level. Despite all its shortcomings, methylene blue remains the standard viability stain most likely due to its availability.
Availability: easily available and inexpensive.
Trypan Blue – also a blue dye that stains dead cells blue. Unlike methylene blue, this stain’s action is based on cell membrane integrity. It is a negatively charged compound that can’t cross the membrane unless it has been damaged. Dead cells have ruptured membranes, allowing the dye to enter and stain them while living cells are very selective in respect to what goes in or out and trypan blue is not allowed to enter. This stain is not dependent on chemical reactions, gradients, reduction states etc., which makes it the gold standard for non-fluorescent vital dyes. It should be noted that it is toxic and cells exposed to it for too long will die and stain blue. Other two stains in this study will be compared to it to measure their effectiveness.
Availability: not easily available to homebrewers and expensive.
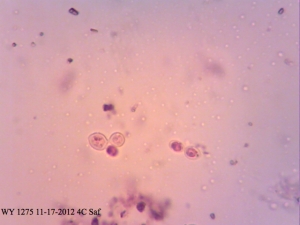
Safranin – a red-pink dye that is often used in histology and cytology to stain nuclei as well as the last stain in the gram staining procedure. Not many people know that it’s also often used as a vital dye in microbiology (often together with methylene blue) and stains dead cells red.
Availability: easily available and inexpensive.
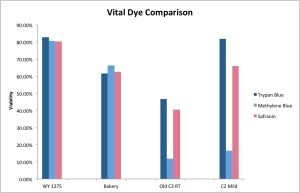
In this post I’ll test these three stains to assess viability of a couple of yeast strains and see how well they correlate. Trypan blue will be used as the standard with methylene blue and safranin compared to it to assess staining accuracy. While it is common knowledge that, despite its faults, methylene blue can be used to check Saccharomyces viability with some degree of accuracy, there isn’t much about using it to look at Brettanomyces. With growing interest in Brettanomyces, both in homebrewing as well as industry, it is important to have a reliable means of assessing culture viability, especially since all-Brett beers require at least a twofold increased pitch compared to regular ales. Checking these stains with some Brettanomyces cultures was therefore the logical thing to do.
Here you can see some micrographs of the various stains in Brettanomyces and Saccharomyces. For this study I looked at 5 cultures:
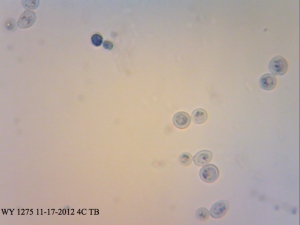
WY 1275 Thames Valley – washed yeast cake from a primary that has been stored in the fridge for over 2 months. Very interesting how viable the cells are! According to Mr. Malty’s calculator, the viability of this slurry should have been 10%.
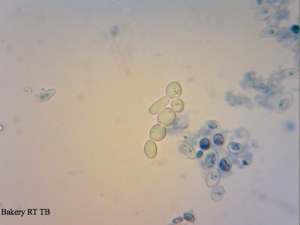
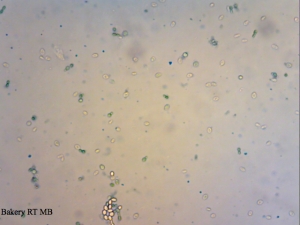
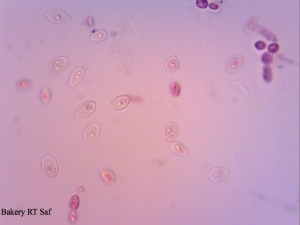
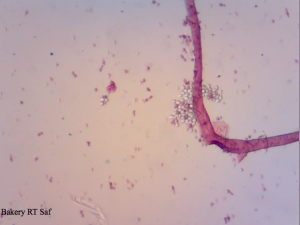
A yeast sample I got from a local bakery – mixed culture with 2 or 3 yeast strains and some Lactobacillus floating around.
Cantillon Iris C3 isolate – a bit of that yeast strain that’s been sitting at room temperature since the time I was sending out these cultures to you guys.
No good pictures, sorry
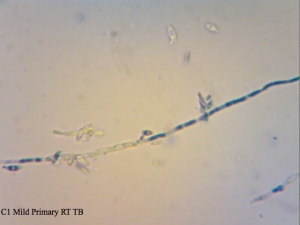
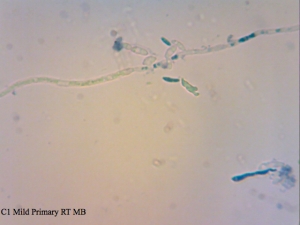
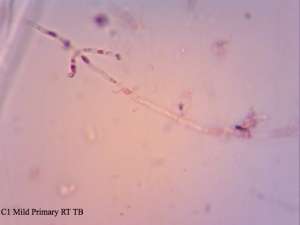
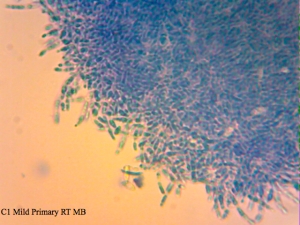
Cantillon Iris C2 isolate – sample pulled from an all-Brett mild that’s currently in primary. Micrographs say C1 on them, and that’s a typo. I made sure to take cells from the pellicle and it appears that some of them are dead, while the ones from the liquid are at near 100% viability (the last photo in this series). The total viability was calculated from a homogenized sample – meaning both liquid and pellicle mixed together.
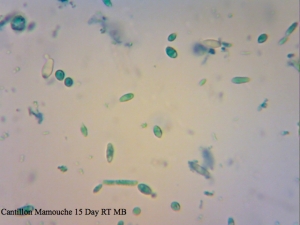
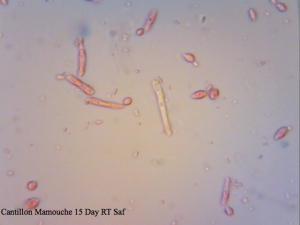
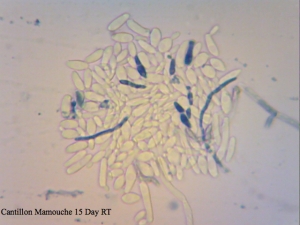
Cantillon Mamouche Dregs – straight dregs from a bottle of Mamouche that I left to sit at room temperature for 15 days before sampling to allow the organisms to wake up a little before trying to culture anything from it. The viability is so low that staining was essentially meaningless and so was not used to determine efficiency.
I found it especially curious how trypan blue and safranin can differentially stain cells in clumps while methylene blue has a bit more trouble differentiating between live and dead ones. It’s also curious that the “filamentous” forms of Brettanomyces that we often see in old cultures and dregs, but very seldom in active ones are stained as dead. It makes sense though. Perhaps that form is the final stage in the nutrient poor environment, which is why we see them in old cultures, and they eventually die afterwards. Another cool thing is that unlike methylene blue, trypan blue and safranin don’t stain living bacteria, which means it can be used to assess the viability of your lactic acid bacteria cultures. This is something that’ll have to be followed up on.
As a result of this little staining series, it appears that methylene blue and safranin are good for assessing Saccharomyces viability, but not Brettanomyces. While safranin seems to be more accurate for Brettanomyces than methylene blue, it still isn’t as good as trypan blue. At least it somewhat approximates the actual viability. The error in counts may be too great to actually be useful so the search for a reliable Brettanomyces stain that’s readily available still goes on. If any of you have had a different experience with these stains please let me know. It is entirely possible that my results do not reflect every Brettanomyces strain out there.
There are a couple more stains I’m interested in looking at, but that’s for another post. Once again sorry for not posting more often, but I’ve just been so very busy that I can’t even brew. It looks like the research is getting into its rhythm and I should be able to manage my time better in the upcoming weeks. Hopefully soon enough the blog will be back to normal with timely posts.
Thanks for posting this.
I have pretty much abandoned MB staining to test the viability of a culture due to the fact that it is not all that reliable and because I try to avoid pitching yeast that is more than a week old. But based on this article I may want to get some safranin. Is that stuff safe to handle?
When I looked into explanations why MB doesn’t work so well I found this: http://voices.yahoo.com/the-methylene-blue-staining-procedure-yeast-viability-3911512.html which says that MB may not be able to enter dead cells and thus leads to an overestimation of viability. That has also been my experience when I tested an old culture, found ~20% viability with an MB stain but nothing grew on a plate. You seem to be indicating the opposite. I.e. that MB may enter and stain living cells.
And don’t worry about posting less often. I prefer quality over quantity anyway.
Hey Kai!
It is pretty safe. As far as I know your biggest concern is staining your hands pink. I got mine from HomeScienceTools and it’s a 1% aqueous solution. Here is the data sheet (http://www.hometrainingtools.com/images/art/CH-SAFRAN.pdf) Frankly I was a bit surprised when I saw it used as a vital stain in some papers and wanted to try it.
MB does seem like it’s not very good indeed.
Also, check out page 11 of this article printed in Methods in Enzymology in 2002. I posted it over at Woodland’s blog in the recent yeast post, but sometimes my comments don’t go through.
Click to access startedyeast.pdf
I used safranin in the lab before to stain bacteria cells. I did this in a direct comparison with crystal violet because I wanted to get away from the toxic and messy crystal violet. I came to the conclusion that in my case safranin works as well as crystal violet.
I have CV, but haven’t tried it as a vital stain (though I read that it is). Perhaps I will some other time too 🙂
Dmitri, thank you very much for sharing your latest results. Really interesting thought to stain Brettanomyces. I have never thought about that. I only use MB at home to stain the cells. Seem to work for Saccharomyces but never tried it with Brettanomyces so far. I am currently doing some Brett work myself and I could easily stain some Brett strains to give you more data.
Cool that your baker’s yeast include some Lactobacillus strains as well. I thought this is only the case in Switzerland. By the way the Lactobacillus in the baker’s yeast seem to improve the quality of the bread. That’s what a Master student found out at my previous University. And we could taste a lot of bread for this study 😉 The bread with pure Saccharomyces was not that “bready” compared to a bread made with the Lactobacillus baker’s yeast. As far as I know, they even identified the strains to be Lactobacillus plantarum, Lactococcus lactis ssp. lactis, Leuconostoc mesenteroides and Lactobacillus brevi. However, I don’t know if this is true for every baker’s yeast.
@Kai: Thank you for sharing your MB experiences as well. In my opinion, only plating is a valid method to determine the viability of a yeast population. On the other hand plating takes some time and I guess staining the cells with MB is a good estimation of viability. And maybe a reason why it is common in yeast labs. I guess most of the people stain relatively viable yeast cells anyway. Therefore taking a method to its extreme might not lead to conclusive results.
I agree with Kai, don’t worry about not posting on a regular basis.
Cheers, Samuel
Hi, I’m completely new to microscopy and cell biology and have arrived here because I am a home brewer and have subscribed to your blog for the advice you give. My apologies for such a simple question but I really don’t know where to start. I have some methylene blue and have tried staining (following instructions) so I can do a cell count on my yeast. My problem to date is that the stains are far too dark. Can you point me at some instructions that will show me how to use the stain properly so I can achieve the same clarity of your photos above?
It could be that your stain is too concentrated. What is the concentration?
It could also be that your microscope’s diaphragm is closet and you could try opening it to let more light through.
I don’t know its concentration, I will take a look at the bottle. What would you expect it to say?
Something like “Mythylene Blue 1% aqueous solution”
Try also looking at your microscope’s diaphragm.
Great post! I’m glad some one else is seeing that viability doesn’t always decrease with storage in the refrigerator as much as some of the calculators indicate.
For your stain do you add Glycine to help with the clumping?
Why would I add glycine?
Awesome post, thanks for your work! About that Cantillon C3 isolate – with no good pictures – do you recall approximately what the viability looked like now?
It’s in the graph at the end of the post. Around 60%.
Jeff,
Have you tried alkaline methylene violet? It’s been shown to have very good results in both viability and vitality. It’s kind of a bitch to prep but I can send you some if you want to try it out. It’s what we use here in the brewery. Probably not practical for home brewers.
Thomas.
I’m using a pre-prepared alkaline methylene violet from white labs but my cells don’t seem to be taking up the stain. I see some very pink debris but even in very old samples I’m only seeing clear or somewhat dark cells. Do you have any suggestions?
I just picked up a microscope off a friend and grabbed some slides and methylene blue to do some viability tests on my yeast. I note from the graph that its performance with Saccharomyces strains (which is all I use) isn’t much different to trypan blue. I did a stain test in the past day or two for some practice at the method, with some yeast that had been stored at ~20C for about a week, which came out at 85.6% viability with MB staining (1830 total cells counted, 264 stained). Have yet to test a refrigerated yeast but will be doing that next week; that one has been in the fridge for 5 months, so no idea what the results of that test will be. I’ve also managed to find some trypan blue online for a reasonable price and am planning to buy it and do a similar side by side test against MB with the same yeast culture to see how close they are in my case.
This post has been quite educational, and while I’m only really doing this for fun/out of curiosity, I’m always keen to learn more about brewing and all the parts that make it up.
Cheers 🙂
So, on a whim, I decided to see if I could use run-of-the-mill food coloring. It worked! I tried both water-based and oil-based (“candy color”). Water-based was slightly more effective.
Pingback: The 5 Steps In The Process Of Wine Making | Wine Spotter
Pingback: The Hemacytometer And A Few Notes On Distillery Microscopy – Boston Apothecary