On April 10, 2011, I made a 15 gal batch of Berliner. Never really thought of making this style until the friendly folks at Brooklyn Homebrew gave me a couple old WYeast 3191, and since this one requires no boil I went ahead and made a big batch.
As per WYeast description “This blend includes a German ale strain with low ester formation and a dry, crisp finish. The Lactobacillus included produces moderate levels of acidity. The unique Brettanomyces strain imparts a critical earthy characteristic that is indicative of a true Berliner Weisse. When this blend is used, expect a slow start to fermentation as the yeast and bacteria in the blend is balanced to allow proper acid production. It generally requires 3-6 months of aging to fully develop flavor characteristics. Use this blend with worts containing extremely low hopping rates.”
The first 5 gal were kegged and consumed in about a month and it was tart and refreshing for sure. The remaining 10 were split in half. One went on top of a can of Oregon Raspberry puree (in spirit of the fact that it is often consumed with Raspberry syrup), and the other was just left as is. Pellicles came and went, and the beers got funky and clear.
Today I noticed that the pellicle is back on the plain one and since my home lab is almost set up, I decided to take a look at it.

As you can see, a certain organism dominates the composition of the pellicle. Based on the size and shape it isn’t Saccharomyces cerevisiae nor is it Lactobacilli (the pellicle looks different), and it sure doesn’t look like Acetobacter to me. This leaves only Brettanomyces unless some organism from the grain managed to thrive and overtake everything else in the brew which is not very likely IMO.
There are a number of other organisms present as well. Tiny motile bacteria are slithering around, a few round yeast cells (most likely Saccharomyces) have been observed, and it just looks like a very “lively and wild” beer.
Since I was at it, I decided to swirl up the raspberries with a racking cane in the other half just to make sure all the fruity goodness gets into the final beer as well as take a look at it under microscope. The pellicle on that one is long since gone so I expected to see a lot of Brett at the bottom. Not so! By far the major organism down there was Saccharomyces and quite a few of them were budding. This is unexpected. The beer has the same funk as it’s brother, but with a definite raspberry presence.
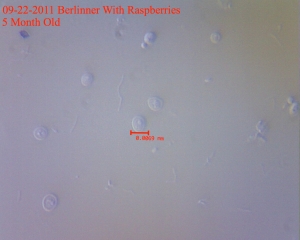 You can see yeast budding on top and on the right. The long filaments are the raspberries. Some bacteria in there as well.
You can see yeast budding on top and on the right. The long filaments are the raspberries. Some bacteria in there as well.
It is also VERY interesting to see ascospore formation in Saccharomyces. I have never seen them with my eyes before today and actually didn’t even realize what I was looking at. For those who don’t know, this is another way of yeast reproduction. Haha! Bet you thought yeast reproduces by budding, right? Well this is the “post budding” stage when the cell produces 4 or 8 copies of its chromosomes and packages them into these spores. These spores can later develop into new yeast. I don’t know much about this as I am not a mycologist so if anyone out there knows about this, feel free to share.
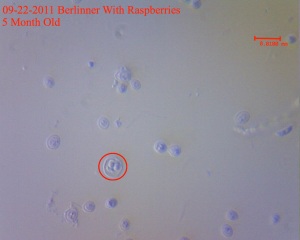 You can see them really well in this micrograph
You can see them really well in this micrograph
 Pretty cool huh?
Pretty cool huh?
WY3191 seems like a solid limited release blend liked by everyone who tried it. Since it is only available about once every other year, I feel very good about the fact that I saved 10mL of the original in my fridge. As soon as I finish my setup (or at least the current stage of it) I’m going to take a look at it, plate, and reculture the Sacch, Brett, and Lacto. As for the beers, I’ll give the plain version 2 more weeks before bottling, and maybe a month or two to the other one for everything to settle down again.









