Some time ago I surfed the net for some beer science articles, but left it off due to just being too busy. Then, a recent email exchange with one of my readers regarding yeast breeding and selection rekindled my interest in research in the field of brewing. Upon digging around through the ever-expanding databases of online research articles, I was pleasantly surprised by the amount of science that’s being done in malting, hop and yeast research all directly aimed at brewing. There are journals specifically dedicated to beer science. Some of them are freely accessible while others require subscriptions. Luckily, I can get my hands on most of them.
Since reading even the free articles is not an easy task for those who don’t read scientific literature regularly, I hatched the following idea: I’m going to try reading a beer science article, digest it, and write a brief summary in every day terms for the benefit of the homebrewing community. I’m going to try to make it a regular thing if you, my readers, think it necessary and educational and I’m not just wasting my time and brain power.
Since the conversation that sparked this idea was about yeast selection and breeding, we’re going to review an article on the state of yeast selection and engineering in the brewing industry. The following article was published in Cerevisia in 2012.
Cerevisia
Belgian Journal of Brewing and Biotechnology
Journal of the Associations of Former Students of Belgian Schools of Brewing Sciences
Cerevisia provides a medium for the publication of both full-length articles and short communications on all aspects of malting and brewing, including biotechnology. The Journal will accept papers ranging from genetic or molecular biological aspects to those covering biochemical, chemical or bioprocess engineering aspects, provided that in each case the material is relevant to malting and brewing.
Editor-in-Chief: Jean-Marie Rock
Taken from http://www.journals.elsevier.com/cerevisia
The article is titled “Selecting and Generating Superior Yeasts for the Brewing Industry” and it’s by a group from Belgium. The tone is rather defensive and tries to convince the reader that genetically modified (GM) yeast is not from the devil, but is rather something that may become very useful and beneficial in the brewing world.
That’s about enough introduction, so let’s jump straight into it!

Fermentation has been utilized for food and beverage production for well over 8000 years, and had historically been spontaneous. Such processes resulted in inconsistent results and it was not until 19th century that using single pure yeast for fermentation became a viable idea. While this approach gave much more consistent results, producers now faced a new problem – selecting yeast strain that carried the desirable characteristics. Tools to do that were unavailable until just a few decades ago and so yeast used in breweries and wineries today have more historic rather than scientific reasons. With advent of molecular biology and genetics it has become possible to select or even make yeast strains with “custom tailored” traits.
From the dawn of agriculture humans have been selectively breeding superior livestock and harvesting seed to use for planting resulting in an unnatural, but beneficial to us, evolution known as artificial selection. Its impact on the species subjected to it is very profound. Many modern crops yield harvests that are often over tenfold higher than their wild progenitors. Many such species, especially animals, have been selectively bred to an extent that they wouldn’t be able to survive in nature. One of the most striking examples is the Belgian Blue cattle that is so muscular that calves are too thick to pass through the birth channel and Caesarian section is required for every birth.
Due to differences in size between microbes and cattle, their selective breeding is far behind compared to the latter. While people bred animals for about 10,000 years, it was not until 1680s that we even became aware of existence of microbes thanks to microscopy pioneer Antoni van Leeuwenhoek. It took yet another two centuries for people to even start thinking about involvement of microbes in food production as well as diseases. Louis Pasteur was the first person to show the correlation between various microbes and the corresponding flaws they contributed to wine. Even after it was established that yeast are responsible for characteristics of fermented products, selective breeding was impossible because, unlike cattle, you can’t you take two yeast cells and breed them. Advent of technology that would allow us to do that took place only a few years ago and is still not widely available.
Genetic modification is a recent technique that has already found its way into improving microbes, crops and livestock. Without going into too much detail, genetic modification is very similar to selective breeding. A desired gene is inserted into the DNA of an organism and the resulting GM entity now carries it. Unlike selective breeding, though, the genes are not randomly recombined and genes are not restricted to those of species that can sexually reproduce. For example, cotton plants now carry a gene from a bacterium Bacillus thuringiensis, which produces a compound that’s lethal to parasites while being harmless to humans. This serves two purposes – higher yields of cotton and reduced pesticide use. 77% of soy, 26% of corn (which is by the way a product of millennia of selective breeding, original “corn” being about 2 inches in size) and 21% of canola worldwide are genetically modified.
Even though our understanding of molecular genetics is nowhere near as deep as some people would like to portray it, our techniques are constantly being refined, resulting in more precise and efficient ways of modifying the genome. The newborn field of Synthetic Biology has already shown that it’s possible to construct fully functional genome from scratch and “tailor-made” genes are no longer science fiction (Fig 1). ***Here I want to clarify that “tailor-made” genes does not mean genes that do what you want, i.e. turn sugar into hop alpha acids. It means that we can select a known gene from a library of sequences and insert it precisely where we want to. Activity of genes is very heavily influenced by where they are positioned on the genome as well as the environment. We’re not going to go into discussion about promoters, enhancers, chromatin states, non-coding RNAs and other players in mindblowingly complex world of epigenetics. An example of “tailor-made” gene would be inserting a gene encoding a fluorescent protein precisely after some other gene. That way they are both expressed if the original gene is active allowing us to track it. Example below.***
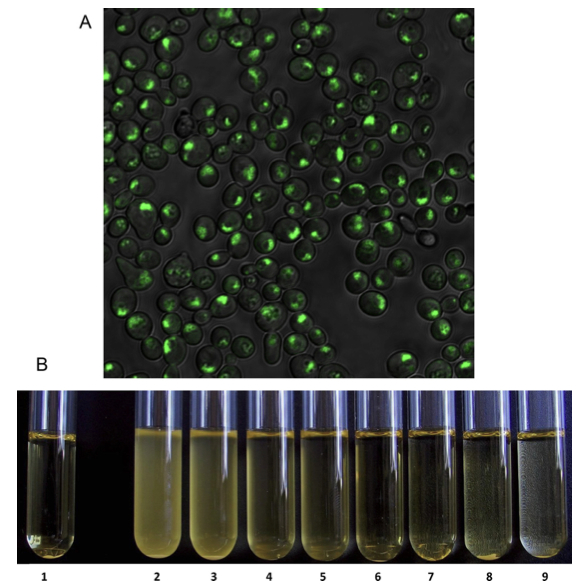
Fig. 1. Examples of experiments to improve industrially relevant phenotypes in yeast strains. (A) Genetically modified yeasts in which one of the most important genes for aroma production is fused to a gene which codes for a green fluorescent protein (GFP). This way, the enzyme that accounts for the production of aroma compounds lights up green, so we are able to study its activity and localization in the cell. (B) Genetic modification of a yeast strain to fine-tune the level of flocculation. 1: control, 2–9: gradual increase of flocculation.
Steensels, et al. Cerevisia, 2012
In recent decades there was a rush to create genetically modified organisms for industrial applications which resulted in wonderful inventions like the transgenic safflower seeds that accounts for majority of world’s pro-insulin production and saves millions of diabetics worldwide. There were successes in the food industry, but food scandals, mad cow disease, coupled to ill-informed population resulted in their disappearance and they were eventually forgotten. Despite the initial defeats, GM organisms are slowly taking over and about 10% of crops worldwide are modified.
In recent years several research teams have developed yeast strains that could potentially be very useful in brewing industry. There are now genetically modified strains of yeast that produce less diacetyl, that can ferment dextrins and starch, produce specific flavors etc, but brewers refuse to use them due to bad hype of GM organisms. These strains were often developed to meet specific industrial needs. Strains that can ferment dextrine and starch were developed for the purposes of creating low calorie beers because the resulting beers would have much less residual sugar. Despite commercial interest these beers were never commercialized due to pressure from protest groups and consumer fear of GM organisms. Food scandals and protests resulted in cessation of funding for development on GM bacteria and yeast for industrial and commercial use and the field dwindled to obscurity by late 90s.
Very recently food industry started recognizing benefits of GM yeast once again. One of the latest victories for GM was patenting of a yeast strain in the US that is able to break down certain undesirable aroma compounds in wine with few winemakers already using it in North America. With increased interest, money once more started flowing into yeast development and research teams are now working on making strains to meet specific needs in beer, wine, chocolate, biofuel and other industries. There are already strains that produce various fruity compounds, ferment quickly, ferment completely, produce high or low levels of alcohol, flocculate more or less, able to survive in high alcohol, or even genetically tagged to be distinguished from other strains easily. Though no GM yeasts can be used industrially at this time, it is anticipated to change in coming years and some breweries are already showing interest in such strains.
While we’re waiting for official permissions to use GM yeast, scientists are also working on selecting superior yeasts by means other than genetic modification. This is essentially done the same way it’s done with cattle, only on a much smaller scale. Individual yeast cells are picked, genetically screened to see what genes are up and down and thus determined which ones are better for this or other application, and are then crossed with other yeasts that possess some other desirable characteristics to result in an even more superior strain. Results of such breeding are already in use in our daily lives. Bakers can now choose yeast strains from a whole array of strains each with different characteristics and profiles such as fermentation speed, dough rising and temperature tolerance. Since these strains were developed by selective breeding, they are not GM organisms and can therefore be freely used.
Paradoxically, the biggest roadblock on the road to using GM yeast in brewing is the beer itself. Since yeast contributes such a major portion of aroma and flavor to the beer, brewers are reluctant to change their house strain for something new. It may be somewhat ironic because the traditional brewing strains were mostly selected by trial and error based on the “whatever works better” criterion. That may be the case with old traditional breweries with many years of tradition and consistency behind them, but new breweries and new generation of brewers are less afraid of taking risks, experimenting and making scientifically informed decisions. There are already collaborations between researchers in Belgium and some Belgian breweries to perform large scale screenings to select and breed strains beneficial to brewing industry via non GM means. Some strains that were selected from such screenings are already in industrial use. This, of course is not only limited to beer and Saccharomyces. Other species of yeast that are of importance for food industry, for example chocolate production, are undergoing the same process.
Yeast are grown in variety of different conditions and their industrially relevant characteristics in these conditions are mapped (Fig 2).

Fig. 2. Graphical representation (heat map) of different characteristics of industrial yeast strains. Every row consists of data from a different yeast strain, every column is a different characteristic. “Yellow” is a low score, and “red” is a high score for this certain characteristic. The dendrogram on the left represents the genetic relatedness of the yeasts, based on an AFLP fingerprint exploiting transposon TY1 insertion site polymorphisms. The colour code on the top right indicates the origin of the yeast strains. This kind of analysis allows us to select yeasts with specific beneficial traits, for example to use in industry, or for breeding.
Steensels, et al. Cerevisia, 2012
Analysis of these maps allows for picking of strains with exactly the characteristics desired. Mating different strains is a powerful approach to select for traits like alcohol tolerance. Rather than mating two strains, researchers now utilize an approach called “genome shuffling” where several strains are mated at the same time. This results in millions of strains in the same flask (essentially each cell is its own strain that’s different from others) with a mosaic genome consisting of a mix of the parent strains. Repeating this several times results in progeny with complex traits. In the beginning the parental strains are irradiated to create mutations and thus more genetic diversity. They are then mixed and allowed to mate, after which the ones with some particular desirable trait (like alcohol tolerance) are selected out and further analyzed to assess their other characteristics. This is repeated several times to obtain superior strains. ***At this point I feel it’s necessary to point out that there is more to mating yeast than just taking WY1056 and WY3711, mixing them together and getting a hybrid strain (though you WILL get some hybrid cells, but they’ll be outnumbered 500 billion to one so you’ll never even know they’re there). As far as I know, sporulation (sexual reproduction) is induced so each strain creates spores that can then interbreed with other spores and create hybrid strains. Usually, like in a starter or your fermenter, yeast reproduce asexually by budding. So each cell in the culture is essentially a clone of every other cell so under such conditions they can’t interbreed because there is no breeding. It’s just making copies of yourself.***
Researchers recognize the importance of spontaneous fermentations and are working of creating superior bred blends of yeasts and bacteria to be used for processes like cocoa fermentation in chocolate making. They aim to mimic the complexity and characteristics of spontaneous fermentation while bringing the consistency and superiority of selectively bred microbial strains. ***I just can’t help but think of WY Lambic/Roeselare blends and the possibility of these custom made “superior spontaneous bug blends” that could be used in future brewing.***
With all these scientific advances in both genetic modification as well as selective microbial breeding, brewing and wine industry are still reluctant to try them. Other than government sanctions, tradition, bad publicity and consumer fears play important roles in preventing the use of “superior” microbes on industrial scale. While the GM taboo has almost completely disappeared in medical and agricultural fields, food industry has yet a long way to go. Should they adopt these new strains for the custom made ones? Benefits and dangers of using GM microbes in food are out of scope of that paper.
Some remarks and personal thoughts:
When I said “there are groups working” on this or that throughout this article, those groups often include the one who wrote the original paper. The authors are the same people who actually generated and bred those strains of yeast that are talked about here.
I couldn’t help but think of an article written by Derek at Bear Flavored Ales about the future of hops as I read and wrote this. He speculates that with all the new hop varieties that are becoming increasingly more fruity and specific in terms of which fruit character they impair we will soon see beers that taste like fruit beers without ever touching fruit. I believe that the combination of the new hop breeds and genetically modified yeast made so that they throw off high amounts of strawberry, or apple, or cherry, or pear, or watermelon, or apricot etc will indeed result in very fruity and interesting beers.
I also believe it’s only a matter of time before GM yeast are finally approved for wide use and they will flood the market and brewing industry. Personally I support it and don’t see any zombie apocalypses or Frankenstein coming out of it based on what little I know on the topic, but it’s not my place to tell you if they’re good or bad. If you believe they’re bad and we should only stick to the original “wild” or “feral” strains, that’s perfectly fine and it’s been done for thousands of years with great success. While most old world and traditional breweries will probably choose to maintain their old cultures and stay true to the quality and traditional flavors particular to those breweries, I think new breweries (especially in USA and Belgium where tradition is good, but novelty is better) will most likely embrace these and give us a myriad of exciting new flavors.
With advances in biology being what they are and an ever-growing number of scientifically educated brewers, I think we are standing at the very doorstep of a brewing revolution the likes of which were never seen in history. With increasing number of brewers embracing Brettanomyces, the yeast species that’s been selected against for as long as beer microbiology existed, and realizing what an amazing depth it can contribute to beer. With new hop breeds appearing every year, each with such an amazing and unique array of flavors and aromas. With increasing variety of malted grains at our disposal, some of which have been brought back from near extinction, adding to the malty complexity of the beverage we’re all so fond of. And now, with advances in biology being such that we can practically custom make yeast with properties that we want, I think just a couple decades from now we’ll think over a pint of “Lemon Garden With a Strawberry Twist Saison”, look back at this time and think how back in those days such beers were only a fantasy.
I claim no authorship of all or any parts of the original paper. The original work was done by and belongs to Jan Steensels, Tim Snoek, Esther Meersman, Martina Picca Nicolino, Elham Aslankoohi, Joaquin F. Christiaens, Rita Gemayel, Wim Meert, Aaron M. New, Ksenia Pougach, Veerle Saels, Elisa van der Zande, Karin Voordeckers, and Kevin J. Verstrepen with all due credit and recognition.
I ask my readers to write a comment or send me an email with their thought on whether or not the Beer Science Literature Review is a worthwhile project or not. Do you think doing these mini reviews would benefit the brewing community? Do you have suggestions or some topics that perhaps I could find some literature on? Please let me know. Personally I really enjoyed working on this post. I feel like it was really beneficial for me and expanded my knowledge and, hopefully, it did for you as well.










